The strategy for the OAIC is to serve as a sustained resource to investigators through a broad range of training and research studies; with a goal to address knowledge gaps in our focus area of optimizing reserve and resilience with an emphasis on translational and interdisciplinary research.

Dr. David Lee recently received an award from the OAIC National Coordinating Center to develop a lipidomics platform using UHPLC/ QToF mass spectrometry. His research will use the newly developed methods to better understand how to leverage lipid metabolism to counteract age-related fatty infiltration in the muscle for improved physical resilience in older adults. Dr. Lee is a faculty member in the Department of Orthopaedic Surgery and Duke Molecular Physiology Institute, and receives support from the Pepper Center for collaboration with a colleague at Wake Forest University.
Developmental Projects
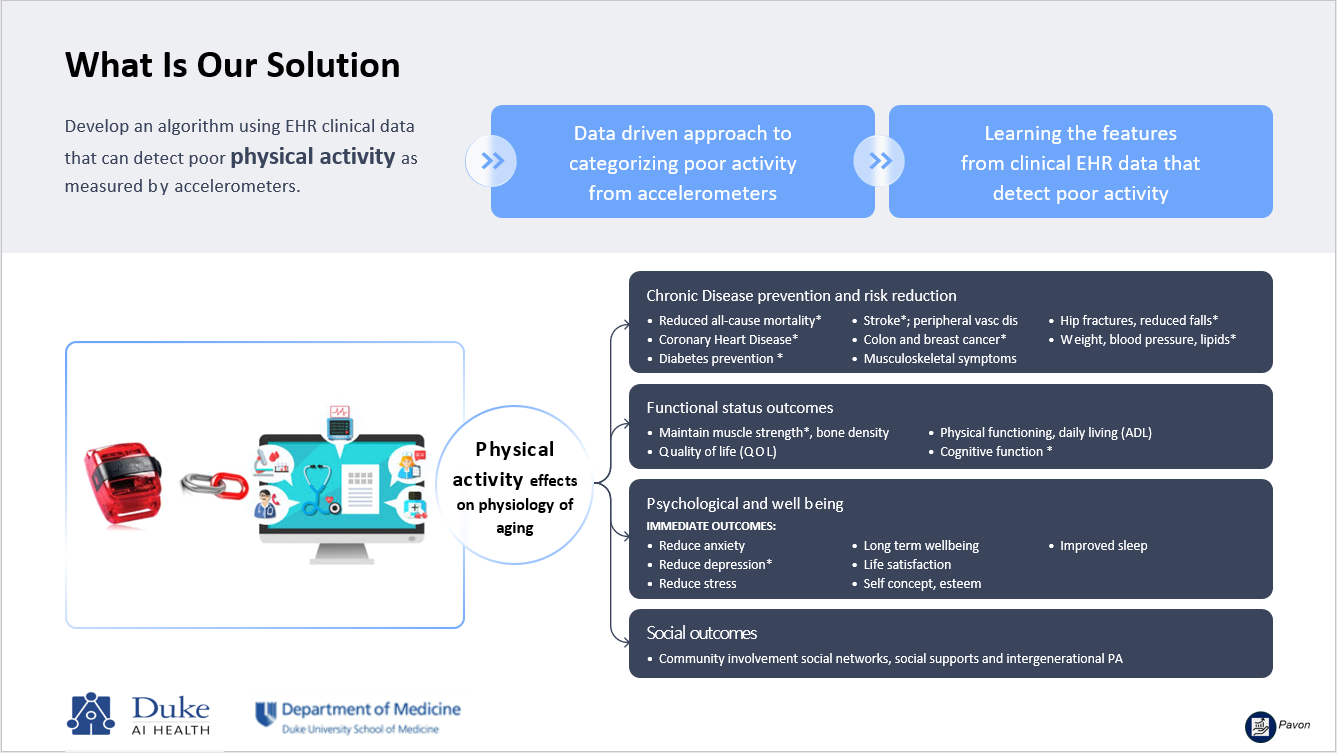
ACCELERATE: Linking ACCELerometer and EHR dATa for Early detection of poor physical activity was a collaboration between Duke Pepper Center and Duke AI Health that leverage electronic health record (EHR) data to create a targeted and scalable approach for the early identification of poor physical activity as an alternative to traditional screening. Led by Juliessa Pavon, MD (Project Leader), the project linked objective physical activity (PA) data measured by wearable accelerometers to patient clinical characteristics and outcomes captured in the EHR. Despite the importance of physical activity metrics for clinical decision-making, PA had not been routinely captured during clinical encounters, nor documented in a discrete or standardized method.
Through the ACCELERATE project, the team harmonized wearable accelerometer data across five Pepper-funded studies, including step counts, activity duration, and activity intensity. Using these harmonized data, the team developed a machine learning-based predictive algorithm leveraging EHR data to accurately classify patients' physical activity levels (sufficient, insufficient, or sedentary). This work built on growing evidence from large epidemiologic studies demonstrating the association between low physical activity and adverse outcomes across major chronic diseases, particularly in populations at high risk for functional decline who frequently go on to receive geriatrics care.
Results from this study provided foundational new knowledge regarding the clinical utility of an EHR-based digital algorithm to detect poor physical activity using objective accelerometer measures. The project demonstrated the feasibility of identifying patients most in need of early geriatrics-focused interventions using remote monitoring, enabling clinicians to proactively tailor care plans to promote healthy aging and resilience. This work aligned with the shared goals of Duke AI Health and the Duke Pepper Center to advance scalable, data-driven approaches to improve care for older adults.
Funding for Dr Pavon’s research was provided by the Duke Department of Medicine Health Data Science Grant Program.
Pavon JM, Fish LJ, Colón-Emeric CS, Hall KS, Morey MC, Pastva AM, Hastings SN. Towards “mobility is medicine”: Socioecological factors and hospital mobility in older adults. J Am Geriatr Soc. 2021 Jul;69(7):1846-1855. PMCID:8273111
Pavon JM, Sloane RJ, Pieper CF, Colón-Emeric CS, Cohen HJ, Gallagher D, Hall KS, Morey MC, McCarty M, Hastings SN. Accelerometer-Measured Hospital Physical Activity and Hospital-Acquired Disability in Older Adults. J Am Geriatr Soc. 2020 Feb;68(2):261-265. PMCID:7002200
Pavon JM, Sloane RJ, Pieper CF, Colón-Emeric CS, Gallagher D, Cohen HJ, Hall KS, Morey MC, McCarty M, Ortel TL, Hastings SN. Physical Activity in the Hospital: Documentation and Influence on Venous Thromboembolism Prophylaxis. J Aging Phys Act. 2020 Apr 24;28(2):306-310. PMCID:7210062
Pavon JM, Sloane R, Morey MC, Hastings SN. Inpatient Mobility Measures As Useful Predictors of Discharge Destination in Hospitalized Older Adults. J Am Geriatr Soc. 2017 Jan;65(1):224-226. PMCID:5832492
The D-FIST is a graded exercise test designed to evaluate cardiorespiratory fitness in clinical populations through a stepping-in-place protocol that requires minimal space and no specialized equipment. Modeled after validated step tests, it accommodates older adults and those with limited mobility or infection isolation restrictions. This developmental project aims to establish the D-FISTS's validity and reliability compared to gold standard cardiopulmonary exercise testing and functional health measures. This project is currently enrolling.
Contact: Amy Pastva
In a typical longitudinal design, follow-up time is predetermined and fixed for every individual to ensure that differences in outcomes can be attributed to true temporal changes rather than variability in measurement timing, and to prevent time-related factors from being conflated with treatment or group effects. While this approach maintains internal validity, it can also limit the ability to explore alternative follow-up schedules that might yield richer information or greater efficiency. We propose a randomized follow-up design, in which the timing of some follow-up measurements is randomized across subjects within a longitudinal study. This approach has the potential to better estimate the underlying trajectory of change. Through a series of simulation studies, we demonstrate that the proposed design can substantially reduce bias when estimating between-group differences and when extrapolating to time points not covered in a fixed-time design. It also improves the overall mean squared error in predicting outcomes across all time points, when higher-order terms in the trajectory cannot be estimated due to insufficient data. This design may be valuable for both clinical trials and prospective observational studies in settings where the trajectory pattern is unknown at the design stage. The promising simulation results motivate further application to real data, to assess the utility of the design in reducing participant burden in data collection while increasing statistical power for trajectory estimation.
Yu, W., Smith, V., & Peskoe, S. (2025, November). Evaluating variable assessment timing design in a simulation study [Poster presentation]. Gerontological Society of America Annual Scientific Meeting, Boston, MA.
Contact: Sarah Peskoe
Through the PERFORM Project, we integrated comprehensive EHR data with advanced machine-learning methods to develop an accurate predictive model of functional decline in older adults, demonstrating strong performance across multiple functional classifications and supporting earlier, more targeted interventions.
Pavon JM, Previll L, Woo M, Henao R, Solomon M, Rogers U, Olson A, Fischer J, Leo C, Fillenbaum G, Hoenig H, Casarett D. Machine learning functional impairment classification with electronic health record data. J Am Geriatr Soc. 2023 Sep;71(9):2822-2833. PMCID:10524844.
Contact: Juliessa Pavon
External Projects
Resilience biomarker and trajectory validation studies (PRIME Knee, SPRING, STEP-HI)
Contact: Cathleen Colón-Emeric
Pragmatic evaluation of events and benefits of lipid-lowering in older adults (PREVENTABLE)
Contact: Kenneth Schmader
Federated evaluation of data to enhance pain research among adults in later life (FEDERAL)
Contacts: Corey Simon and Susan N. Hastings
Expanding Duke Pepper OAIC Molecular Measures Core to offer mass spectrometry-based comprehensive lipidomics
Contact: James Bain
Physical rehabilitation for older adults with acute HFpEF and REHAB-HF trials
Contact: Amy Pastva
Implementation and dissemination of age-friendly health system measures
Contact: Juliessa Pavon
Dynamic biomarkers of pain and function in individuals with osteoarthritis
Contact: Corey Simon
Stem cell metabolism in aging via mass spectrometry-based methods
Contact: David E. Lee
The Physical Performance Across the Lifespan (PALS) Study is a collaboration between the Duke Pepper Center and the MURDOCK Study. Briefly, the PALS Study explores dimensions of health, function, behavior, and physiologic characteristics in a cohort of 1,000 adults aged 30-90+, with oversampling across the older decades. The PALS study includes a comprehensive assessment battery at two time-points: baseline and 2-year follow-up, which concluded in 2018.
The PALS Data Use Request Form streamlines requests for PALS data and facilitates communication between investigators and PALS/Pepper leadership. In addition to specifying what information is needed to make a request, a standardized description of the PALS cohort and publication-related details are provided.
The PALS Data Dictionary is a reference for variable names, field types, and field descriptions for the current subsets of PALS data: Baseline questionnaire, Montreal Cognitive Assessment (MoCA) test battery, Health Self-assessment questionnaire, Physical performance tests, Two-year follow-up questionnaire, Metabolomics assays, and Accelerometer-derived variables.
The PALS Codebook is a comprehensive overview of PALS data and associated MURDOCK data, including statistical summaries and graphs. The Codebook also includes an abbreviated version of the PALS data dictionary.
NOTE: The PALS Codebook will be updated as new data become available. Always check the revision date on the Codebook title page to insure you have the most recent reference.