Duke Pepper Center offers numerous workshops and events each year for researchers, consistent with the Center's mission to understand and optimize reserve and resilience. See below for upcoming research training workshops and events.
Workshops
The Data Integration Working Group (DIWG) is an essential learning laboratory for OAIC investigators. The DIWG integrates basic, translational and clinical research across the OAIC by providing a biweekly multidisciplinary venue for ongoing and proposed science to be reviewed in a “Works-in-Progress” setting. IOC members, REC Scholars, Pilot Awardees, DP leaders and their investigative teams meet to confirm, generate, integrate and interpret data. Within the DIWG venue, the OAIC stimulates new ideas and facilitates translation among basic research, clinical research, and clinical practice. The DIWG also provides Scholars with regular access to Core expertise and learning opportunities. The DIWG is co-led by Drs. Wm. Kraus, Cathleen Colón-Emeric, and Sarah Peskoe.
The DIWG meets during the spring and fall on the second and fourth Thursdays of the month from 9:30am-11am. Meeting dates for the 2025/2026 academic year are listed below; speakers and projects to be discussed will be posted when available. For questions about the group or to participate in group meetings, please contact Dr. Wm. Kraus (william.kraus@duke.edu).
Fall Dates
September 11: Sarah Peskoe, PhD - Reporting Errors and Missing Data in Medical Records
September 25: Bill Kraus, MD - The Value and Availability of Data and Sample Repositories for Aging Research
October 9: Joshua Johnson, DPT, PhD - Enhancing Resilience Among Patients with Stroke: Implementing High Intensity Home-Based Rehabilitation
October 23: Monica Agrawal, PhD - Large Language Models for Data Abstraction from Electronic Health Records
November 13: Neill Li, MD - The Role and Interactions of Macrophages on the Age-Associated Changes of the Neuroimmune Response in Peripheral Nerve Regeneration
December 11: Corey Simon, DPT, PhD - Circulating Biomarkers Associated with Pain Intensity and Interference after Total Knee Arthroplasty: Results from the PRIME-Knee Resilience Study
Spring Dates
January 8: Caroline Sloan, MD, MPH - Models of Care: Medications, EHR, & Polypharmacy
January 22: Chris Mosher, MD, MHS - Investigating Senolytic Properties in Pulmonary Rehabilitation and Metformin in COPD Exacerbations (INSPIRE-COPD-E)
February 12: Cathleen Colón-Emeric, MD, FACP, MHS; Virginia Kraus, MD, PhD; Sarah Peskoe, PhD - The PRIME Study: Predicting Resilience in Elders Undergoing Joint Replacement
February 26: Charles Semelka, MD, MS - Circulating Senescence Biomarkers Predictive of Physical Function and Clinical Outcomes in Patients Hospitalized with Heart Failure: Insights from REHAB-HF
March 12: Thomas Laskow, MD - Immune Reserve and Resilience
March 26: Kathryn Porter-Starr, PhD, RD - Nutrition as Modifiers of Physical Reserve and Resilience
April 9: Leanna Ross, PhD - Enhancing Long-term Reserve into Late Life with a Six-Month Exercise Intervention in Middle Age
April 23: Semra Ozdemir, PhD - TBA
May 14: Corey Simon, DPT, PhD - Primary Results from the ReLOAD Study
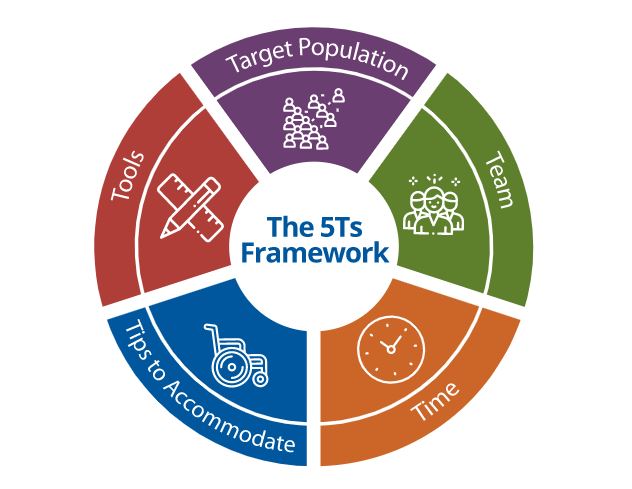
Consulting and Collaborating: Using the 5Ts Framework
Research investigators play critical roles in identifying important research questions, designing research studies, and obtaining funding. The 5Ts Framework provides a systematic approach to help investigators address the NIH Inclusion Across the Lifespan Policy and maximize generalizability of study findings.
Learning objectives:
Researchers, such as principal investigators, co-investigators, and research mentees, visit 5Ts Framework site page for insight. Resources include:
- Preparing grant proposals that are inclusive of older adults by avoiding exclusion criteria that disproportionately affect older adults
- Ensuring that research funding will be adequate to support the inclusion of older adults by budgeting for additional time
- Sharing the 5Ts with your research team by downloading and completing the 5Ts Inclusion Worksheet during a team meeting
- Hear about new developments by reading 5Ts News

The objective of this workshop is to support early investigators in developing and testing complex interventions targeting older populations.
Organized around the Medical Research Council framework for the development and evaluation of complex interventions to improve health, and the NIH Stage Model, participants will have the opportunity to develop or refine their own intervention ideas over 7 sessions. Expert faculty facilitators review key points using case study examples, highlight resources and provide self-study materials. Ample time for discussion, small group work, feedback, and problem-solving around the scholars’ research areas is provided. Topics and learning objectives are listed below.
2026 Workshop Dates
The next workshop will be held in September and October 2026.
REGISTRATION
- Workshop is limited to 25 participants
- Participants must commit to attending at least 5 of the 7 sessions
- Registration will be announced July 2026.
- In the online registration form, you will be asked to attach a current CV or biosketch, and briefly describe the intervention you are considering
Workshop Topics and Objectives
Introduction to Intervention Development Frameworks
Faculty facilitators: Colón-Emeric, Strauman
- List the phases of complex intervention development and describe why they are necessary
- Identify types of complex research in older adults, and why complex interventions are usually required
- Scholars give brief presentations of their intervention area and stage of development
- Review course goals and logistics
Pre-Clinical or Theoretical Phase
Faculty facilitators: Crowley, Zullig, Somers
- Define and quantify the problem, identify and quantify the population most at risk/likely to benefit
- Understand the pathways by which the problem is caused/sustained
- Describe the existing evidence that your proposed intervention might have the desired effect
- List commonly used models of behavior change, organizational change, multiple risk factor reduction, etc. that are useful in aging research
- Adopt, adapt, or develop a theoretical model for your intervention
Phase II: Incorporating Implementation Science Principles
Faculty facilitators: Bosworth
- List CFIR domains and describe how features of each are associated with successful implementation of complex interventions
- Describe methods for identifying and overcoming potential implementation barriers early in intervention development
- List types of implementation measures that are useful during intervention pilot testing
Phase I: Defining components of the intervention
Faculty facilitators: Whitson, Steinhauser, Johnson
- Describe the organizational/social/environmental context for your proposed intervention
- Use the theoretical model to identify critical leverage points on the pathway and potential interventions to change them
- Use principles of community/participant engaged research to obtain stakeholder input into the intervention components and delivery and ensure equity
- Understand how intervention components may need to be adapted for elderly/vulnerable groups, and groups underrepresented in research.
- List basic qualitative methods useful for intervention development and refinement
Phase II: Exploratory Trial – Optimize intervention
Faculty facilitators: Schmader, Pieper
- Describe common pilot study designs
- Measure the feasibility and acceptability of intervention components
- List components of intervention fidelity and how to measure them
- List data you will need for design of an efficacy/effectiveness trial and strategies for obtaining it in the exploratory phase
Phase II: Exploratory Trial – Optimize evaluation
Faculty facilitators: Hall, Ramos
- List types of outcomes commonly examined in aging research
- Identify resources for validated Patient Reported Outcomes
- Use strategies to minimize participant burden
- Identify issues in adapting outcomes for older populations
- Describe how exploratory trials inform optimization of measurement protocols
Phase III: Pragmatic Trial Design
Faculty facilitators: Colón-Emeric, Bowling
- List options for testing a complex intervention and when each is most appropriate: individual randomized trial, group randomized trial, stepped-wedge designs
- Describe principles/pitfalls in estimating sample size requirements for each design type
- Understand human subjects and ethical considerations in pragmatic trials of older adults
The Health Disparities Research Curriculum is designed to increase knowledge and skill in the design and conduct of health disparities research. The curriculum consists of didactic and interactive sessions. See the workshop website for more information.
The Duke Pepper Center hosted the workshop, Remote Patient Monitoring in Action: Connecting Research, Technology, and Care at Duke, in March 2025. See the event website for more details.
Events
Tour the Duke Center for Neurodegeneration and Neurotherapeutics with Andy West
Wednesday, July 30, 2025
5:00 pm - 6:00 pm
3 Genome Court, MRSBIII, Durham, NC 27710
Pop Health DataShare Services and PACE
Speaker: Amy Clark, PhD, Director of the DataShare Service Center, Department of Population Health Sciences
Wednesday, April 16, 2025
5:00 pm - 6:00 pm
Aging Center Hub, Duke South, Blue Zone, Room 2514
The most recent Resilience Research Colloquium was held on February 4, 2025. See the event website for details and the full agenda.